Carbon and Its Compounds – Textbook Exercise Questions
1. Ethane, with the molecular formula C₂H₆ has:
(b) 7 covalent bonds.
An ethane molecule has one single covalent bond between the two carbon atoms (C-C) and six single covalent bonds between the carbon and hydrogen atoms (C-H). This gives a total of 1 + 6 = 7 covalent bonds.
2. Butanone is a four-carbon compound with the functional group:
(c) ketone.
The name “butanone” has two parts: “but-” which means it has four carbon atoms, and the suffix “-one,” which indicates it belongs to the ketone family.
3. While cooking, if the bottom of the vessel is getting blackened on the outside, it means that:
(b) the fuel is not burning completely.
When fuel does not burn completely due to insufficient oxygen, it produces soot, which is unburnt carbon. This soot gets deposited on the bottom of the vessel, making it black.
4. Explain the nature of the covalent bond using the bond formation in CH₃Cl.
A covalent bond is a chemical bond formed by the mutual sharing of electrons between atoms to achieve a stable electron configuration, like that of a noble gas.
In methyl chloride (CH₃Cl), the central carbon atom has four valence electrons and needs four more to complete its octet. It achieves this by sharing electrons with three hydrogen atoms and one chlorine atom.
-
Each hydrogen atom shares its one electron with carbon.
-
The chlorine atom, which has seven valence electrons, shares one of its electrons with carbon.
Through this sharing, carbon forms four single covalent bonds, and all the atoms in the molecule achieve a stable electron configuration.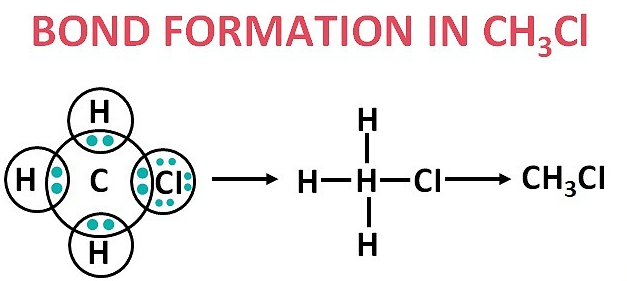
5. Draw the electron dot structures for:
(a) ethanoic acid (CH₃COOH)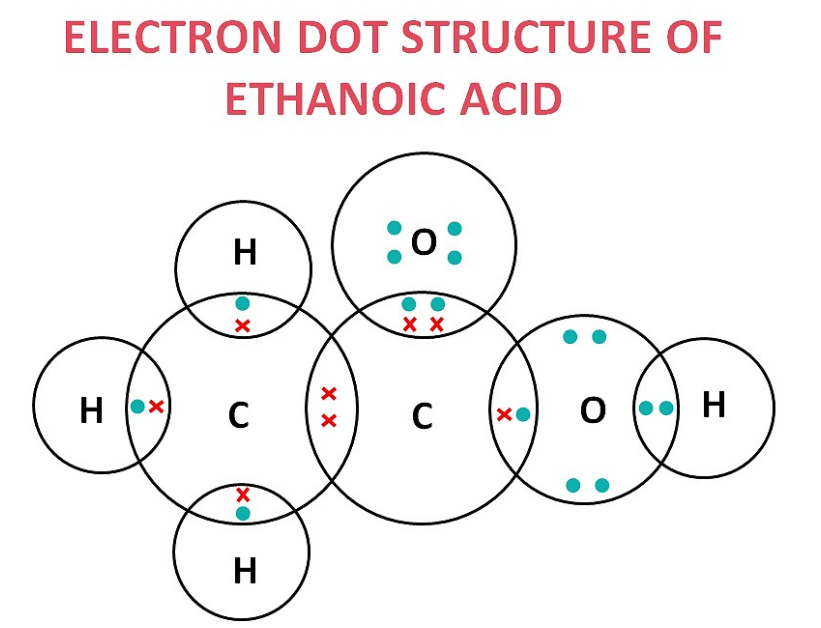
(b) H₂S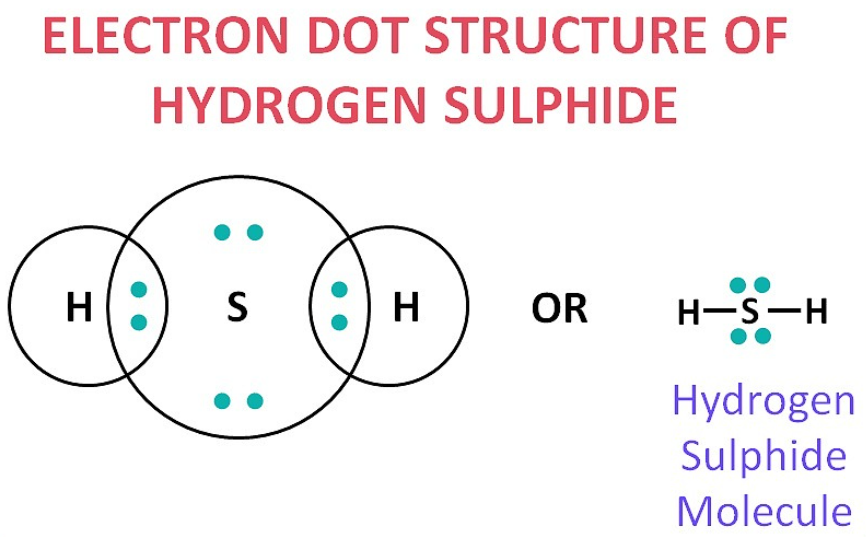
(c) propanone (CH₃COCH₃)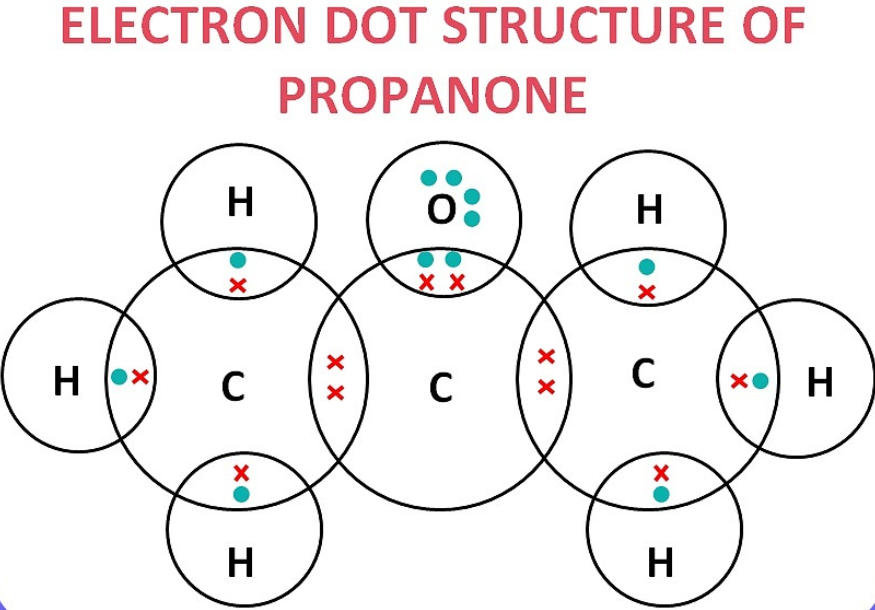
(d) F₂
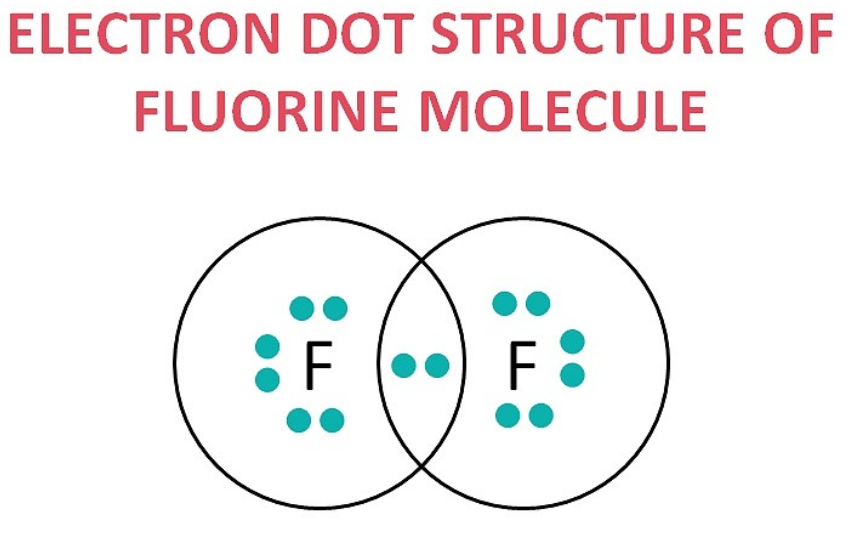
6. What is an homologous series? Explain with an example.
A homologous series is a group of organic compounds that have the same functional group and similar chemical properties. Each member of the series differs from the one before it by a –CH₂ group.
Example: The homologous series of alcohols.
The first three members are:
-
Methanol (CH₃OH)
-
Ethanol (C₂H₅OH)
-
Propanol (C₃H₇OH)
All of these compounds contain the –OH functional group, have similar chemical properties, and each member has one more carbon atom and two more hydrogen atoms than the previous one.
7. How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
| Basis of Difference | Ethanol (Alcohol) | Ethanoic Acid (Carboxylic Acid) |
| Smell | Has a pleasant, sweet smell. | Has a pungent, vinegar-like smell. |
| Litmus Test | Is neutral; does not change the color of litmus paper. | Is acidic; turns blue litmus paper red. |
| Reaction with Sodium Carbonate | Does not react. | Reacts to produce brisk effervescence (fizzing) due to the release of carbon dioxide gas. |
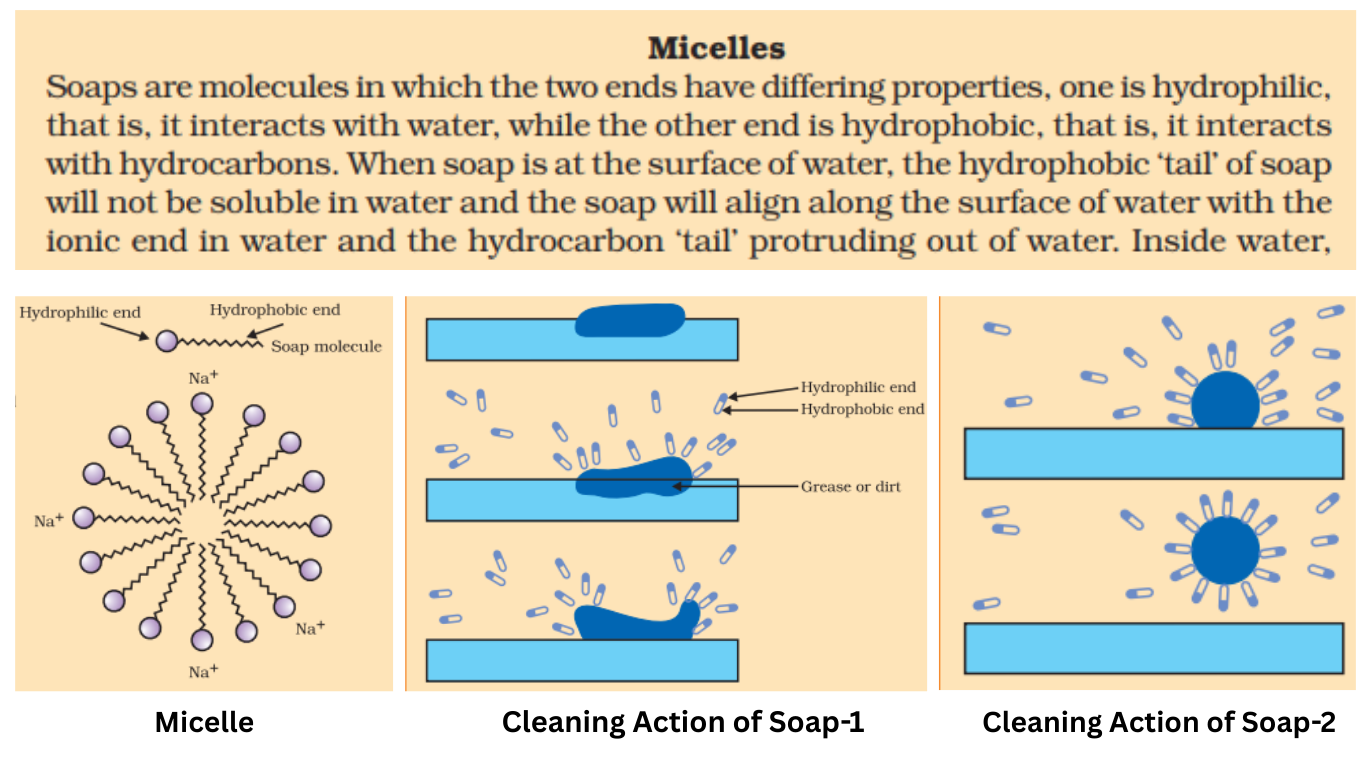
8. Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Soap molecules have two parts: a long hydrocarbon tail that is hydrophobic (water-repelling) and a short ionic head that is hydrophilic (water-attracting).
When soap is dissolved in water, the molecules arrange themselves in a unique way to keep their hydrophobic tails away from the water. They form spherical clusters called micelles, where the tails point towards the center of the cluster, and the hydrophilic heads face outwards, in contact with the water.
A micelle will not be formed in a solvent like ethanol. This is because the hydrocarbon tail is soluble in organic solvents like ethanol, so there is no need for the molecules to form a cluster to “hide” the tail.
9. Why are carbon and its compounds used as fuels for most applications?
Carbon and its compounds are used as fuels for two main reasons:
-
High Calorific Value: They have a high calorific value, which means they release a large amount of heat and energy when they burn.
-
Controlled Combustion: Their combustion can be easily controlled, allowing us to regulate the amount of energy released as needed for different applications like running engines or power plants.
10. Explain the formation of scum when hard water is treated with soap.
Hard water contains dissolved mineral salts, specifically calcium and magnesium ions. Soap molecules are sodium or potassium salts of fatty acids. When soap is used in hard water, the calcium and magnesium ions react with the soap molecules to form an insoluble, sticky precipitate called scum. This scum floats on the water, reduces the cleaning ability of the soap, and sticks to clothes.
11. What change will you observe if you test soap with litmus paper (red and blue)?
Soap solutions are slightly alkaline (basic) in nature. Therefore:
-
There will be no change in the color of blue litmus paper.
-
The soap solution will turn red litmus paper blue.
12. What is hydrogenation? What is its industrial application?
Hydrogenation is a chemical process that involves the addition of hydrogen to an unsaturated hydrocarbon (one with double or triple bonds) to convert it into a saturated hydrocarbon (one with only single bonds). This reaction usually requires a catalyst like nickel or palladium.
Industrial Application: The most common industrial application is the hardening of vegetable oils. Liquid vegetable oils are unsaturated and are converted into solid or semi-solid saturated fats (like vanaspati ghee) through hydrogenation.
13. Which of the following hydrocarbons undergo addition reaction: C₂H₆, C₃H₈, C₃H₆, C₂H₂ and CH₄.
Addition reactions are characteristic of unsaturated hydrocarbons (alkenes and alkynes).
-
C₃H₆ (Propene) is an alkene with a double bond.
-
C₂H₂ (Ethyne) is an alkyne with a triple bond.
The other compounds (C₂H₆, C₃H₈, and CH₄) are all alkanes (saturated) and do not undergo addition reactions.
Therefore, C₃H₆ and C₂H₂ undergo addition reactions.
14. Give a test that can be used to differentiate chemically between butter and cooking oil.
You can use the bromine water test to differentiate between butter (a saturated fat) and cooking oil (an unsaturated fat).
-
Take two test tubes, one with cooking oil and the other with a small amount of melted butter.
-
Add a few drops of bromine water (which is reddish-brown) to each and shake.
-
Observation:
-
In the test tube with cooking oil, the reddish-brown color of the bromine water will disappear. This is because the bromine reacts with and adds across the double bonds of the unsaturated oil.
-
In the test tube with butter, the reddish-brown color will remain as there are no double bonds for the bromine to react with.
-
15. Explain the mechanism of the cleansing action of soaps.
The cleansing action of soap depends on the structure of soap molecules and the micelles they form in water.
-
Most dirt and grease are oily and do not dissolve in water.
-
When soap is applied, the hydrophobic (oil-loving) tails of the soap molecules attach themselves to the oily dirt particle.
-
The hydrophilic (water-loving) heads of the soap molecules face outwards, towards the surrounding water.
-
This forms a micelle with the dirt particle trapped in its center.
-
When the cloth is rinsed with water, the micelles are washed away, carrying the dirt with them and leaving the cloth clean.
InText Questions
1. What would be the electron-dot structure of carbon dioxide which has the formula CO₂?
In CO₂, the carbon atom is in the center. It shares two pairs of electrons with each oxygen atom, forming two double bonds. Each oxygen atom is left with two non-bonding pairs of electrons (lone pairs).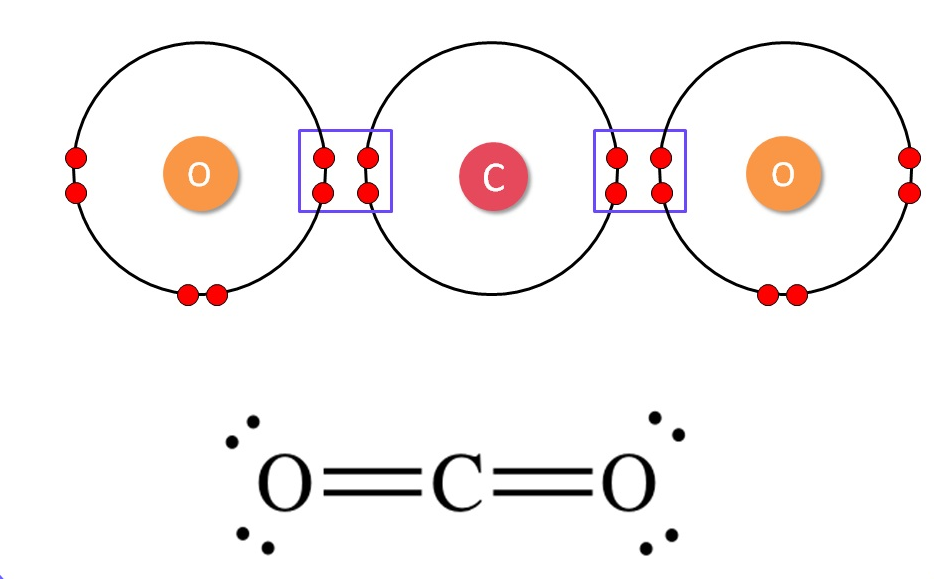
2. What would be the electron-dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? (HINT: The eight atoms of sulphur are joined together in the form of ring.)
The S₈ molecule forms a crown-shaped ring. Each sulfur atom forms a single covalent bond with two neighboring sulfur atoms. To complete its octet, each sulfur atom also has two lone pairs of electrons.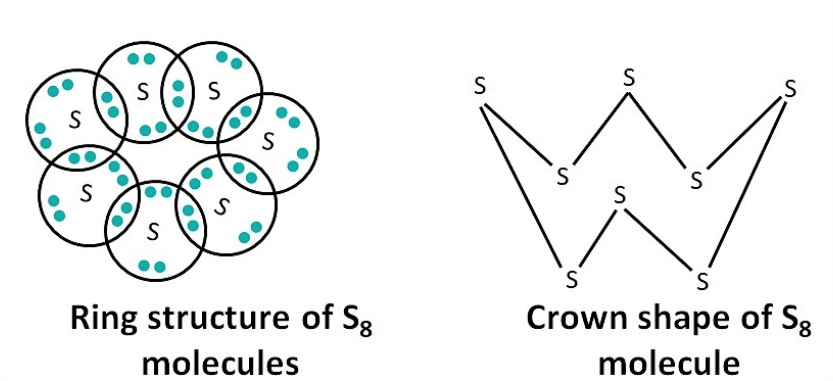
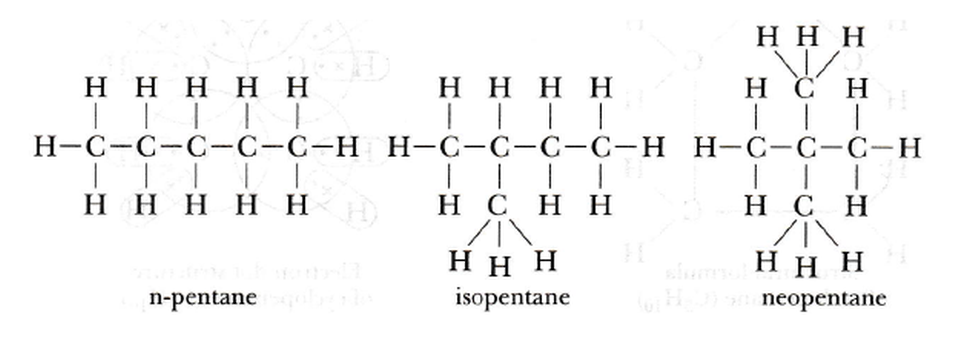
3. How many structural isomers can you draw for pentane?
Pentane (C₅H₁₂) has three structural isomers.
-
n-pentane: A straight chain of five carbon atoms.
-
Isopentane (2-methylbutane): A four-carbon chain with a methyl group attached to the second carbon.
-
Neopentane (2,2-dimethylpropane): A three-carbon chain with two methyl groups attached to the central carbon.
4. What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
-
Catenation: The unique ability of carbon atoms to form strong covalent bonds with other carbon atoms, creating long chains, branched chains, and rings.
-
Tetravalency: Carbon has a valency of four, which means it can form four covalent bonds. This allows it to bond with many other elements in various combinations.
5. What will be the formula and electron dot structure of cyclopentane?
The formula is C₅H₁₀.
The structure is a ring of five carbon atoms, each bonded to two hydrogen atoms.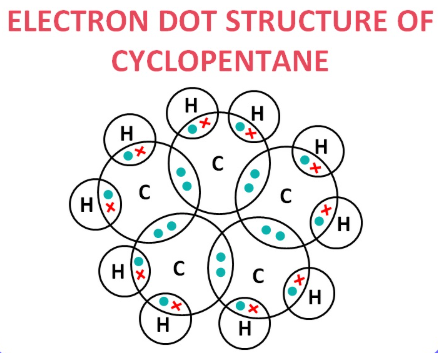
6. Draw the structures for following compounds:
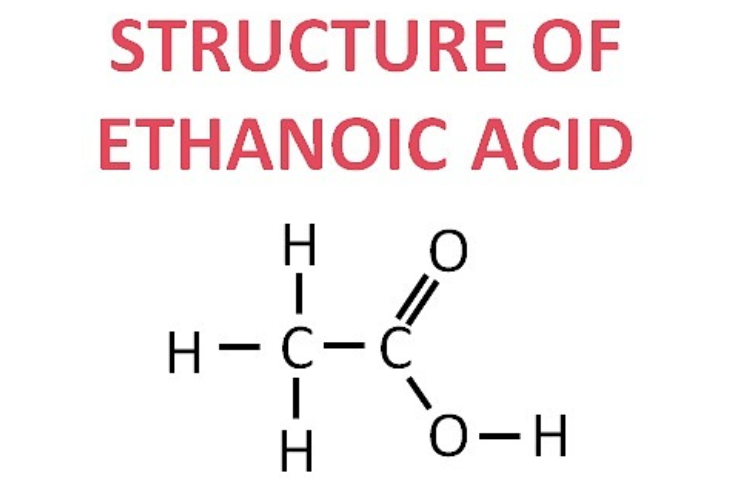
(i) Ethanoic acid: CH₃–COOH
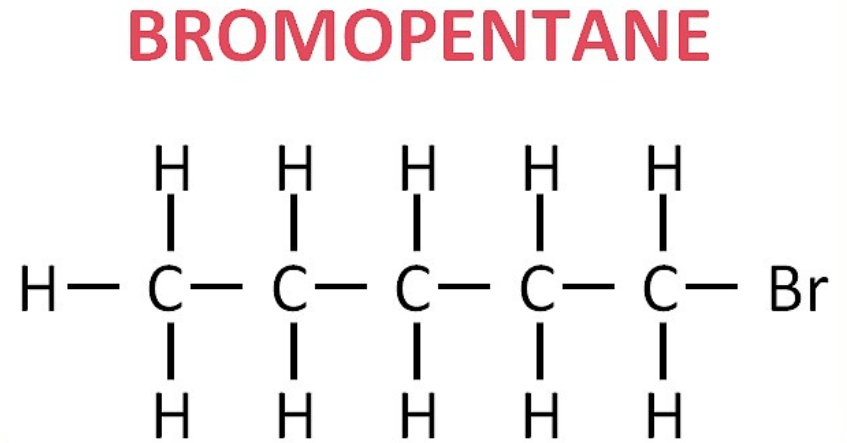
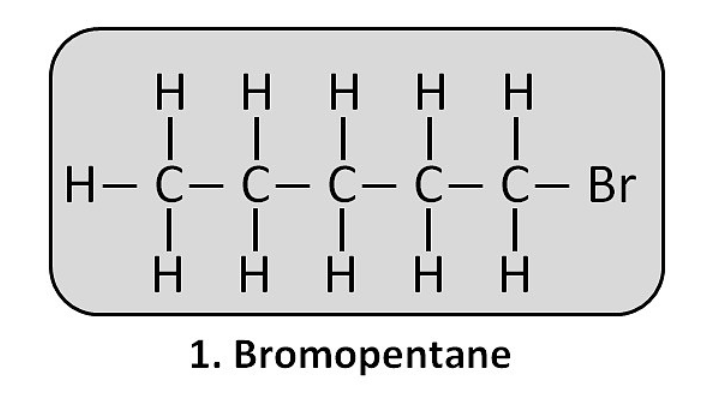
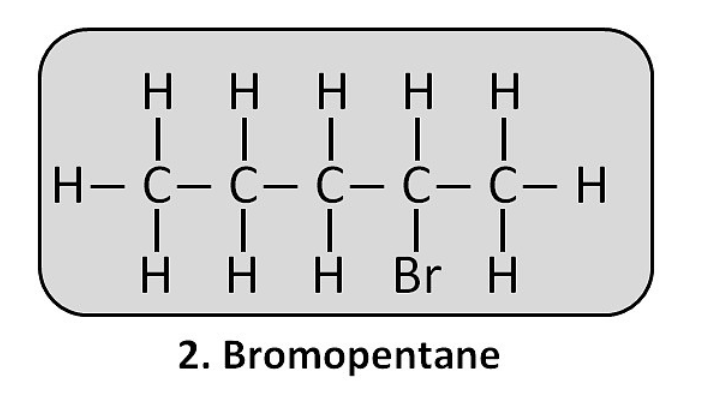
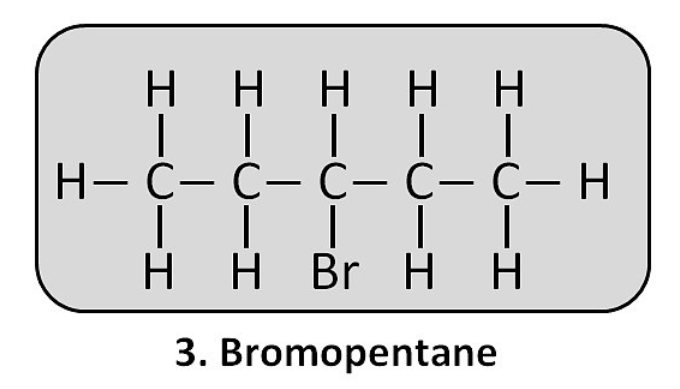
(ii) Bromopentane: CH₃–CH₂–CH₂–CH₂–CH₂–Br (This is 1-bromopentane. Other isomers are also possible).
Are structural isomers possible for bromopentane?
Yes. The bromine atom can be attached to the first, second, or third carbon atom of the pentane chain, creating different structural isomers (1-bromopentane, 2-bromopentane, 3-bromopentane).
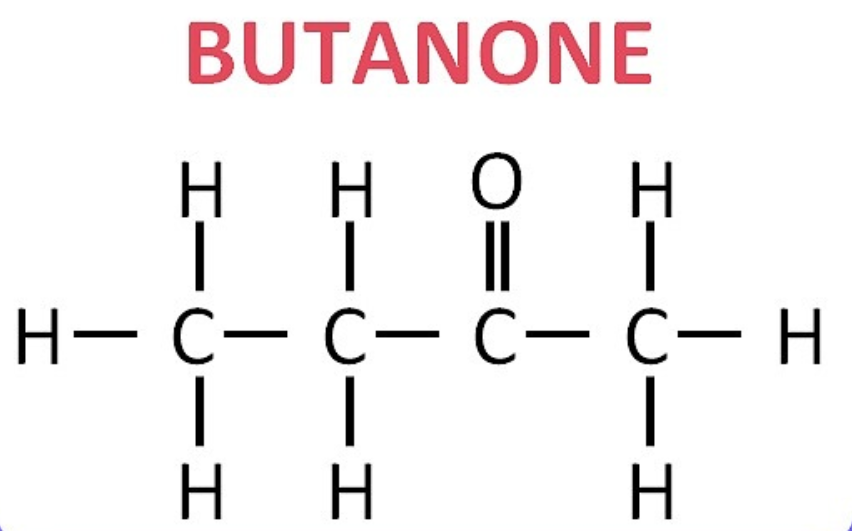
(iii) Butanone: CH₃–CO–CH₂–CH₃
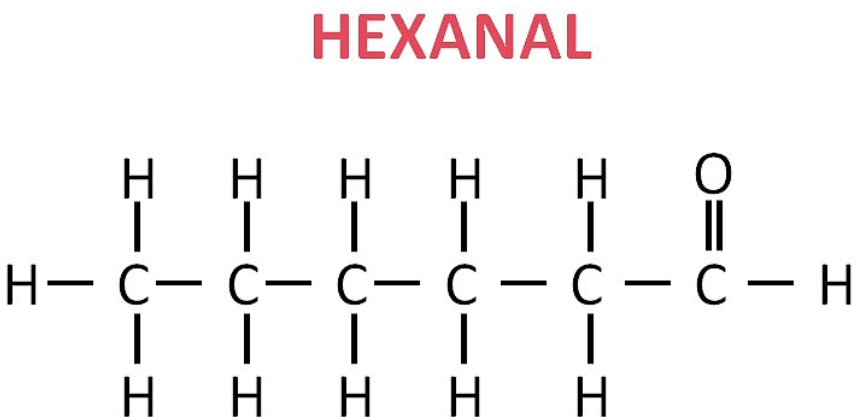
(iv) Hexanal: CH₃–CH₂–CH₂–CH₂–CH₂–CHO
7. How would you name the following compounds?
(i) CH₃–CH₂–Br : Bromoethane
(ii) HCHO : Methanal
(iii) CH≡C–CH₂–CH₂–CH₂–CH₃ : Hex-1-yne
8. Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
It is an oxidation reaction because it involves the addition of oxygen. Ethanol (CH₃CH₂OH) is converted to ethanoic acid (CH₃COOH) by an oxidizing agent, which provides an oxygen atom to the molecule.
9. A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Welding requires an extremely high temperature.
-
Burning ethyne with pure oxygen produces a very hot flame because the combustion is complete and highly concentrated.
-
Air is only about 21% oxygen. If ethyne is burned with air, the combustion is incomplete and less efficient. The large amount of nitrogen in the air absorbs much of the heat, resulting in a cooler flame that is not hot enough for welding.
10. How would you distinguish experimentally between an alcohol and a carboxylic acid?
Use the sodium carbonate test.
-
Add a small amount of sodium carbonate to separate test tubes containing the alcohol and the carboxylic acid.
-
Observation:
-
With the carboxylic acid, you will see brisk fizzing (effervescence) as carbon dioxide gas is released.
-
With the alcohol, there will be no reaction.
-
11. What are oxidising agents?
Oxidising agents are substances that cause another substance to be oxidized. They achieve this by giving oxygen to the substance or by removing hydrogen from it. In the process, the oxidising agent itself gets reduced.
12. Would you be able to check if water is hard using a detergent?
No. Detergents work well in both hard and soft water and produce lather in both. Unlike soap, detergents do not form scum in hard water. Since there would be no noticeable difference in the lather produced, a detergent cannot be used to determine if water is hard.
13. People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is this agitation necessary to get clean clothes?
This agitation is necessary because it provides mechanical force to help remove the dirt. The soap micelles trap the oily dirt, but they need to be physically dislodged from the fabric. Beating, scrubbing, or spinning the clothes helps to knock the micelles loose from the cloth fibers so they can be carried away by the rinse water. This combination of the soap’s chemical action and the physical agitation is what gets the clothes truly clean.