Acids, Bases and Salts – Detailed Summary
This chapter delves into the fundamental concepts of acids, bases, and salts, exploring their properties, reactions, everyday importance, and the pH scale.
1. Introduction to Acids and Bases:
-
Acids: Substances that are sour in taste, turn blue litmus red, and often produce hydrogen ions (H⁺) when dissolved in water. Examples include hydrochloric acid (HCl), sulphuric acid (H₂SO₄), nitric acid (HNO₃), and acetic acid (CH₃COOH).
-
Bases: Substances that are bitter in taste, feel soapy to touch, turn red litmus blue, and often produce hydroxide ions (OH⁻) when dissolved in water. Examples include sodium hydroxide (NaOH), potassium hydroxide (KOH), calcium hydroxide (Ca(OH)₂), and magnesium hydroxide (Mg(OH)₂).
-
Indicators: Substances that change their color in acidic or basic solutions, helping to identify the nature of a substance. Common indicators include litmus (natural), turmeric (natural), methyl orange (synthetic), and phenolphthalein (synthetic). Olfactory indicators change their odor in acidic or basic media (e.g., onion, vanilla essence).
2. Chemical Properties of Acids and Bases:
-
Reaction with Metals:
-
Acids react with most metals to produce a salt and hydrogen gas (H₂).
-
Acid + Metal → Salt + Hydrogen gas
-
-
Bases react with certain metals (like zinc, aluminium) to produce hydrogen gas and a salt containing a complex anion.
-
Base + Metal → Salt + Hydrogen gas (e.g., NaOH + Zn → Na₂ZnO₂ + H₂)
-
-
-
Reaction of Acids with Metal Carbonates and Metal Hydrogencarbonates:
-
Acids react with metal carbonates and metal hydrogencarbonates to produce the corresponding salt, carbon dioxide gas (CO₂), and water.
-
Acid + Metal Carbonate/Hydrogencarbonate → Salt + Carbon Dioxide + Water
-
-
The CO₂ gas produced turns lime water (calcium hydroxide solution) milky due to the formation of insoluble calcium carbonate.
-
-
Reaction of Acids and Bases with Each Other (Neutralization):
-
Acids and bases react with each other to nullify each other’s effect, producing a salt and water. This is called a neutralization reaction.
-
Acid + Base → Salt + Water (e.g., HCl + NaOH → NaCl + H₂O)
-
-
-
Reaction of Metallic Oxides with Acids:
-
Metallic oxides (which are generally basic in nature) react with acids to form salt and water, similar to a neutralization reaction.
-
Metallic Oxide + Acid → Salt + Water
-
-
-
Reaction of Non-metallic Oxides with Bases:
-
Non-metallic oxides (which are generally acidic in nature) react with bases to form salt and water.
-
Non-metallic Oxide + Base → Salt + Water (e.g., CO₂ + Ca(OH)₂ → CaCO₃ + H₂O)
-
-
3. What do all Acids and all Bases have in Common?
-
All acids produce hydrogen ions (H⁺(aq)) when dissolved in water. This H⁺ ion cannot exist alone and combines with water molecules to form hydronium ions (H₃O⁺).
-
All bases produce hydroxide ions (OH⁻(aq)) when dissolved in water.
-
The presence of H⁺(aq) ions is responsible for the acidic properties, and OH⁻(aq) ions for basic properties.
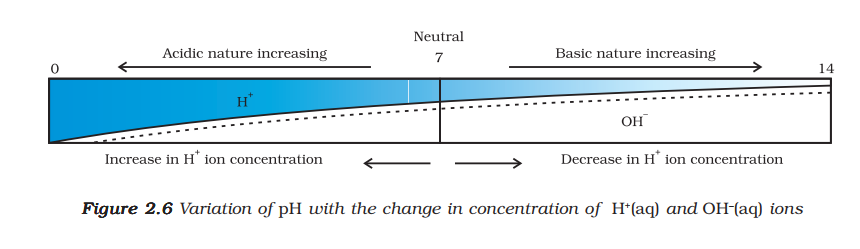
-
Acids or Bases in Water Solution: The process of dissolving an acid or a base in water is highly exothermic. Concentrated acids or bases must always be added slowly to water with constant stirring, never water to acid/base, to prevent splashing and excessive heat generation. This process is called dilution, and it decreases the concentration of H₃O⁺ or OH⁻ ions per unit volume.
4. Strength of Acids and Bases – The pH Scale:
-
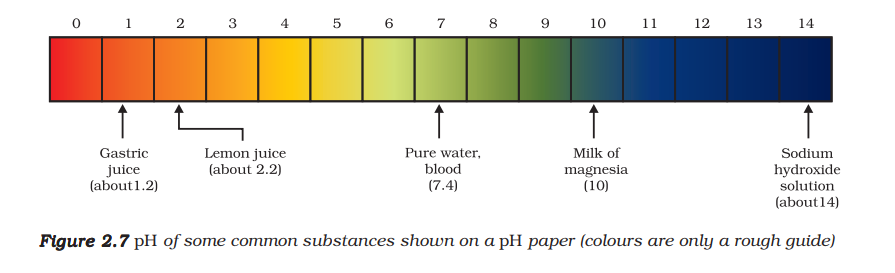
pH Scale: A scale for measuring hydrogen ion concentration in a solution. The ‘p’ in pH stands for ‘potenz’ (German for power). The scale ranges from 0 (very acidic) to 14 (very alkaline/basic).
-
pH = 7: Neutral solution (e.g., pure water)
-
pH < 7: Acidic solution (lower the pH, stronger the acid)
-
pH > 7: Basic/Alkaline solution (higher the pH, stronger the base)
-
-
Universal Indicator: A mixture of several indicators that shows different colors at different pH values, providing a more precise measure of pH than litmus paper.
-
Importance of pH in Everyday Life:
-
Living Organisms: Most living organisms, including humans (blood pH ~7.35-7.45) and plants, function within a narrow pH range. Acid rain (pH < 5.6) is harmful.
-
Soil pH: Plants require a specific soil pH for healthy growth. Farmers adjust soil pH using substances like quicklime, slaked lime, or chalk if it’s too acidic.
-
Digestive System: Our stomach produces hydrochloric acid (pH ~1.5-3.5) to aid digestion. Excess acid causes indigestion, which is relieved by antacids (mild bases like Milk of Magnesia – Mg(OH)₂).
-
Tooth Decay: Caused when mouth pH falls below 5.5. Bacteria degrade sugars, producing acids that corrode tooth enamel (calcium hydroxyapatite). Using basic toothpastes neutralizes these acids.
-
Self-defense by Animals and Plants: Bee stings inject methanoic acid, causing pain. Applying a mild base like baking soda provides relief. Nettle leaves have stinging hairs that inject methanoic acid. Dock plant leaves (often found nearby) are basic and can neutralize the sting.
-
5. More About Salts:
-
Family of Salts: Salts having the same positive or negative radicals belong to the same family (e.g., NaCl and KCl are chloride salts; NaCl and Na₂SO₄ are sodium salts).
-
pH of Salts:
-
Salts of strong acid and strong base: Neutral (pH = 7) (e.g., NaCl)
-
Salts of strong acid and weak base: Acidic (pH < 7) (e.g., NH₄Cl)
-
Salts of weak acid and strong base: Basic (pH > 7) (e.g., CH₃COONa)
-
-
Chemicals from Common Salt (NaCl):
-
Sodium Hydroxide (NaOH): Produced by the electrolysis of an aqueous solution of NaCl (brine) – known as the Chlor-alkali process. Products are NaOH, Cl₂, and H₂.
-
Uses of Cl₂: Water treatment, PVC, disinfectants.
-
Uses of H₂: Fuels, margarine, ammonia for fertilizers.
-
Uses of NaOH: Degreasing metals, soaps, detergents, paper making.
-
-
Bleaching Powder (CaOCl₂): Produced by the action of chlorine gas on dry slaked lime (Ca(OH)₂).
-
Uses: Bleaching cotton/linen, wood pulp; oxidizing agent; disinfecting drinking water.
-
-
Baking Soda (Sodium Hydrogencarbonate – NaHCO₃): Produced using NaCl, H₂O, CO₂, and NH₃. It’s a mild non-corrosive basic salt.
-
Uses: Making baking powder (mixture of NaHCO₃ and a mild edible acid like tartaric acid), ingredient in antacids, in soda-acid fire extinguishers. On heating, it produces Na₂CO₃, H₂O, and CO₂.
-
-
Washing Soda (Sodium Carbonate Decahydrate – Na₂CO₃·10H₂O): Obtained by recrystallizing sodium carbonate (produced by heating baking soda). It’s a basic salt.
-
Uses: Glass, soap, and paper industries; manufacture of borax; cleaning agent; removing permanent hardness of water.
-
-
-
Water of Crystallization: The fixed number of water molecules present in one formula unit of a salt. Salts containing water of crystallization are called hydrated salts (e.g., CuSO₄·5H₂O – blue vitriol; Na₂CO₃·10H₂O – washing soda; CaSO₄·2H₂O – gypsum). Heating these crystals removes the water, and the salt may change color and become amorphous.
-
Plaster of Paris (Calcium Sulphate Hemihydrate – CaSO₄·½H₂O): Obtained by heating gypsum (CaSO₄·2H₂O) at 373 K (100°C), causing it to lose water molecules. It’s a white powder that, on mixing with water, sets into a hard solid mass (gypsum).
-
Uses: Supporting fractured bones, making toys, decorative materials, making surfaces smooth.
-
The chapter concludes with a summary of key concepts and exercises to reinforce understanding.
List of Activities in Tabular Form
| Activity Number | Title/Aim of the Activity | Brief Description of Procedure |
| 2.1 | Testing substances with indicators (litmus, phenolphthalein, methyl orange). | Collect samples (HCl, H₂SO₄, HNO₃, CH₃COOH, NaOH, Ca(OH)₂, etc.) and test with red/blue litmus, phenolphthalein, and methyl orange solutions. Record color changes. |
| 2.2 | Testing olfactory indicators. | Test finely chopped onion, vanilla essence, and clove oil with dilute HCl and dilute NaOH. Observe changes in odor. |
| 2.3 | Reaction of zinc granules with dilute sulphuric acid. | Add zinc granules to dilute H₂SO₄ in a test tube. Observe gas evolution. Test the gas with a burning splinter (popsound indicates H₂). |
| 2.4 | Reaction of sodium carbonate/hydrogencarbonate with dilute HCl. | React Na₂CO₃ and NaHCO₃ with dilute HCl. Pass the gas evolved through lime water. Observe lime water turning milky. |
| 2.5 | Testing the reaction of acids and bases (neutralization). | Add phenolphthalein to NaOH solution (turns pink). Add HCl dropwise until the pink color disappears. Then add NaOH again. |
| 2.6 | Reaction of metallic oxide (copper oxide) with acid. | Take copper oxide in a beaker and add dilute HCl slowly while stirring. Observe the color change of the solution (blue-green). |
| 2.7 | Reaction of non-metallic oxide (CO₂) with base (Ca(OH)₂). | (Reference to Activity 2.5 where CO₂ reacted with Ca(OH)₂). |
| 2.8 | Testing solutions for electrical conductivity. | Set up a circuit with a bulb, battery, and beaker. Test solutions like dilute HCl, H₂SO₄, glucose, alcohol, NaOH, Ca(OH)₂ for conductivity. |
| 2.9 | Action of dry HCl gas on dry and wet litmus paper. | Prepare HCl gas. Test its action on dry blue litmus paper and then on moist blue litmus paper. |
| 2.10 | Investigating dilution of acid/base. | Add concentrated H₂SO₄/NaOH pellets to water in a beaker. Observe temperature change. |
| 2.11 | Testing pH of various solutions. | Test pH of saliva (before/after meal), lemon juice, aerated drink, carrot juice, coffee, tomato juice, tap water, NaOH, HCl using pH paper/universal indicator. |
| 2.12 | Determining the pH of soil. | Mix soil with water, filter, and test the pH of the filtrate using universal indicator paper. |
| 2.13 | Identifying the family of salts. | Write chemical formulae of given salts (Potassium sulphate, sodium sulphate, etc.) and identify acids/bases they are formed from and their families. |
| 2.14 | Determining the pH of salt solutions. | Dissolve various salts (NaCl, KNO₃, AlCl₃, etc.) in distilled water and test their pH using pH paper. Identify if they are acidic, basic, or neutral. |
| 2.15 | Investigating if crystals of salts are really dry (water of crystallization). | Heat copper sulphate crystals in a dry boiling tube. Observe color change and water droplets. Add water to the residue. |
| Group Activity I | Prepare your own indicator (e.g., beetroot extract). | Crush beetroot, extract juice, filter. Test the extract with lemon juice, soda-water, vinegar, baking soda solution. |
| Group Activity II | Preparing a soda-acid fire extinguisher. | Suspend an ignition tube with dilute H₂SO₄ in a wash-bottle containing NaHCO₃ solution. Tilt to mix and direct the CO₂ discharge on a burning candle. |
Acids Bases and Salts – Textual Questions
Answers for Page 2 (Question at the top):
This page introduces acids, bases, and how we test them.
-
You have been provided with three test tubes. One of them contains distilled water and the other two contain an acidic solution and a basic solution, respectively. If you are given only red litmus paper, how will you identify the contents of each test tube?
-
Answer: Imagine you have three mystery drinks and only a piece of red litmus paper (which is like a special color-changing strip). Here’s how you’d figure out what’s in each:
-
Dip the red litmus paper into each test tube one by one.
-
The test tube where the red litmus paper turns BLUE: That’s your basic solution (like soap water).
-
Now you have identified the basic solution. Take a piece of the now blue litmus paper (that you just dipped into the basic solution) and dip it into the remaining two test tubes.
-
The test tube where this blue litmus paper turns RED: That’s your acidic solution (like lemon juice).
-
The test tube where the red litmus paper (from step 1) didn’t change color AND the blue litmus paper (from step 3) also didn’t change color: That’s your distilled water (which is neutral, neither acidic nor basic).
-
-
Answers for Page 6 (QUESTIONS section):
-
Why should curd and sour substances not be kept in brass and copper vessels?
-
Answer: Curd (dahi) and other sour foods (like pickles or lemon juice) have acids in them. Brass and copper are metals. When acids react with these metals, they can form harmful, poisonous substances that can get into your food and make you sick. That’s why it’s better to keep sour foods in glass, stainless steel, or ceramic containers.
-
-
Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
-
Answer: When an acid mixes with most metals, hydrogen gas is usually released (it bubbles out).
-
Example: If you put a piece of zinc metal (like from an old battery casing, but be careful!) into dilute hydrochloric acid (a common lab acid), you’ll see bubbles.
-
Zinc (metal) + Hydrochloric acid → Zinc chloride (a salt) + Hydrogen gas
-
-
How to test for hydrogen gas: If you bring a burning matchstick or a lit candle near the bubbles, the hydrogen gas will burn with a “pop” sound. It’s like a tiny explosion!
-
-
Metal compound A reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride.
-
Answer:
-
“Effervescence” means bubbling.
-
If a gas “extinguishes a burning candle,” it means the gas is carbon dioxide (CO₂). (CO₂ doesn’t let things burn).
-
Since calcium chloride (CaCl₂) is formed, the metal compound A must contain calcium.
-
Acids react with metal carbonates to produce CO₂. So, compound A is likely Calcium Carbonate (CaCO₃). This is what chalk, marble, and limestone are made of.
-
-
Equation in simple words: Calcium carbonate + Hydrochloric acid → Calcium chloride + Water + Carbon dioxide gas
-
Chemical Equation: CaCO₃(s) + 2HCl(aq) → CaCl₂(aq) + H₂O(l) + CO₂(g)
-
Answers for Page 9 (QUESTIONS section):
-
Why do HCl, HNO₃, etc., show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character?
-
Answer: Acids like HCl and HNO₃ show acidic behavior (like turning blue litmus red) only when they are dissolved in water. This is because in water, they break apart and release hydrogen ions (H⁺). These H⁺ ions are what make something acidic.
Alcohol and glucose also have hydrogen in them, but when they dissolve in water, they don’t release H⁺ ions. So, they don’t act like acids.
-
-
Why does an aqueous solution of an acid conduct electricity?
-
Answer: When an acid dissolves in water, it breaks up into charged particles called ions (like H⁺ ions and other negative ions). These freely moving ions can carry an electric current through the solution, just like electrons carry current in a wire. So, the acid solution can conduct electricity.
-
-
Why does dry HCl gas not change the colour of the dry litmus paper?
-
Answer: Dry HCl gas doesn’t have any water. For HCl to show its acidic properties (like changing the color of litmus paper), it needs to release H⁺ ions. It can only do this when it’s dissolved in water. Since both the HCl gas and the litmus paper are dry, no H⁺ ions are formed, and the litmus paper doesn’t change color.
-
-
While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
-
Answer: Mixing a strong acid with water creates a lot of heat.
-
If you add acid to water slowly, the water can absorb this heat gradually, and it’s safer.
-
If you add water to acid, so much heat can be produced all at once that the mixture can boil, splash out, and cause serious burns. The glass container might even break! So, always remember: “Acid to Water, like you oughta!”
-
-
-
How is the concentration of hydronium ions (H₃O⁺) affected when a solution of an acid is diluted?
-
Answer: Hydronium ions (H₃O⁺) are basically what H⁺ ions become in water – they are what make a solution acidic. When you dilute an acid (meaning you add more water to it), you are spreading out those hydronium ions. So, the concentration (how many H₃O⁺ ions are in a certain amount of solution) decreases. The acid becomes less strong.
-
-
How is the concentration of hydroxide ions (OH⁻) affected when excess base is dissolved in a solution of sodium hydroxide?
-
Answer: Sodium hydroxide (NaOH) is a base, and it releases hydroxide ions (OH⁻) in water, which make the solution basic. If you dissolve more sodium hydroxide (excess base) into a solution that already has sodium hydroxide, you are adding more OH⁻ ions. So, the concentration of hydroxide ions increases. The solution becomes more strongly basic.
-
Answers for Page 12 (QUESTIONS section, just before “2.4 MORE ABOUT SALTS”):
-
You have two solutions, A and B. The pH of solution A is 6 and pH of solution B is 8. Which solution has more hydrogen ion concentration? Which of this is acidic and which one is basic?
-
Answer:
-
The pH scale goes from 0 to 14. Lower pH means more acidic (more H⁺ ions). Higher pH means more basic.
-
Solution A (pH 6): This is acidic (because pH is less than 7). It has more hydrogen ion (H⁺) concentration than solution B.
-
Solution B (pH 8): This is basic (because pH is more than 7).
-
-
-
What effect does the concentration of H⁺(aq) ions have on the nature of the solution?
-
Answer: The concentration of H⁺ ions (hydrogen ions in water) decides if a solution is acidic, basic, or neutral.
-
Lots of H⁺ ions: The solution is acidic.
-
Very few H⁺ ions (and more OH⁻ ions): The solution is basic.
-
Balanced H⁺ and OH⁻ ions: The solution is neutral (like pure water).
-
-
-
Do basic solutions also have H⁺(aq) ions? If yes, then why are these basic?
-
Answer: Yes, even basic solutions have some H⁺ ions, but they have many, many more hydroxide ions (OH⁻). It’s the much higher amount of OH⁻ ions compared to H⁺ ions that makes the solution basic. Think of it like a seesaw – if the OH⁻ side is much heavier, it’s basic.
-
-
Under what soil condition do you think a farmer would treat the soil of his fields with quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate)?
-
Answer: Quick lime, slaked lime, and chalk are all basic substances. A farmer would add these to the soil if the soil is too acidic. Plants don’t grow well if the soil is too acidic. Adding these basic substances helps to neutralize the extra acid and bring the soil pH to a better level for crops.
-
Answers for Page 17 (QUESTIONS section, at the end of the chapter summary “What you have learnt”):
-
What is the common name of the compound CaOCl₂?
-
Answer: The everyday name for CaOCl₂ is Bleaching powder.
-
-
Name the substance which on treatment with chlorine yields bleaching powder.
-
Answer: To make bleaching powder, you need to react chlorine gas with Slaked lime (calcium hydroxide, Ca(OH)₂).
-
-
Name the sodium compound which is used for softening hard water.
-
Answer: The sodium compound used to make hard water soft is Washing soda (sodium carbonate, Na₂CO₃·10H₂O).
-
-
What will happen if a solution of sodium hydrogencarbonate is heated? Give the equation of the reaction involved.
-
Answer: If you heat a solution of sodium hydrogencarbonate (Baking soda), it breaks down into Sodium carbonate, Water, and Carbon dioxide gas.
-
Chemical Equation: 2NaHCO₃ (heat) → Na₂CO₃ + H₂O + CO₂
-
-
Write an equation to show the reaction between Plaster of Paris and water.
-
Answer: When you mix Plaster of Paris with water, it turns back into a hard solid called Gypsum.
-
Chemical Equation: CaSO₄·½H₂O + 1½H₂O → CaSO₄·2H₂O
-
Question Answers – Main Exercise
Okay, let’s tackle all 15 questions from the “EXERCISES” section at the end of the “Acids, Bases and Salts” chapter (which starts on page 34 of the PDF, which is page 18 according to the chapter’s internal numbering).
Here are the answers in easy language with examples:
EXERCISES (End of Chapter Questions)
-
A solution turns red litmus blue, its pH is likely to be
(a) 1 (b) 4 (c) 5 (d) 10-
Answer: If red litmus turns blue, the solution is basic. Basic solutions have a pH greater than 7.
-
Answer: (d) 10
-
-
A solution reacts with crushed egg-shells to give a gas that turns lime-water milky. The solution contains
(a) NaCl (b) HCl (c) LiCl (d) KCl-
Answer:
-
Egg-shells are made of calcium carbonate (CaCO₃).
-
A gas that turns lime-water milky is carbon dioxide (CO₂).
-
Acids react with carbonates to produce CO₂.
-
From the options, HCl (Hydrochloric acid) is an acid.
-
-
Answer: (b) HCl
-
Example reaction: CaCO₃ (egg-shell) + 2HCl → CaCl₂ + H₂O + CO₂ (gas)
-
-
10 mL of a solution of NaOH is found to be completely neutralised by 8 mL of a given solution of HCl. If we take 20 mL of the same solution of NaOH, the amount HCl solution (the same solution as before) required to neutralise it will be
(a) 4 mL (b) 8 mL (c) 12 mL (d) 16 mL-
Answer: If you double the amount of NaOH (from 10 mL to 20 mL), you’ll need to double the amount of HCl to neutralize it.
-
So, 8 mL (for 10 mL NaOH) * 2 = 16 mL (for 20 mL NaOH).
-
Answer: (d) 16 mL
-
-
Which one of the following types of medicines is used for treating indigestion?
(a) Antibiotic (b) Analgesic (c) Antacid (d) Antiseptic-
Answer: Indigestion is often caused by too much acid in the stomach. To treat it, you need something that neutralizes the acid. “Ant-acid” literally means “against acid.”
-
Answer: (c) Antacid
-
Example: Milk of Magnesia, Eno.
-
-
Write word equations and then balanced equations for the reaction taking place when –
(a) dilute sulphuric acid reacts with zinc granules.-
Word Equation: Zinc + Dilute Sulphuric acid → Zinc sulphate + Hydrogen gas
-
Balanced Equation: Zn(s) + H₂SO₄(aq) → ZnSO₄(aq) + H₂(g)
(b) dilute hydrochloric acid reacts with magnesium ribbon.
-
Word Equation: Magnesium + Dilute Hydrochloric acid → Magnesium chloride + Hydrogen gas
-
Balanced Equation: Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
(c) dilute sulphuric acid reacts with aluminium powder.
-
Word Equation: Aluminium + Dilute Sulphuric acid → Aluminium sulphate + Hydrogen gas
-
Balanced Equation: 2Al(s) + 3H₂SO₄(aq) → Al₂(SO₄)₃(aq) + 3H₂(g)
(d) dilute hydrochloric acid reacts with iron filings.
-
Word Equation: Iron + Dilute Hydrochloric acid → Iron(II) chloride + Hydrogen gas
-
Balanced Equation: Fe(s) + 2HCl(aq) → FeCl₂(aq) + H₂(g)
-
-
Compounds such as alcohols and glucose also contain hydrogen but are not categorised as acids. Describe an Activity to prove it.
-
Answer: Just because something has hydrogen doesn’t mean it’s an acid. Acids release H⁺ ions when dissolved in water, and these ions help conduct electricity. Alcohols and glucose don’t do this.
-
Activity to Prove:
-
Set up a simple circuit with a beaker, two carbon rods (electrodes), a battery, a bulb, and connecting wires.
-
First, pour dilute hydrochloric acid (an acid) into the beaker. The bulb will glow, showing that the acid solution conducts electricity.
-
Now, clean the beaker and rods. Pour a solution of glucose (or alcohol) into the beaker.
-
Try to light the bulb. The bulb will not glow. This shows that glucose (or alcohol) solution does not conduct electricity, meaning it doesn’t produce H⁺ ions in water and therefore isn’t an acid.
-
-
-
Why does distilled water not conduct electricity, whereas rain water does?
-
Answer:
-
Distilled water is very pure water. It has almost no dissolved salts or impurities, so it doesn’t have many ions (charged particles) to carry electricity.
-
Rain water, as it falls through the atmosphere, dissolves gases like carbon dioxide (CO₂) and sulphur dioxide (SO₂). These gases react with water to form weak acids (like carbonic acid, H₂CO₃). These acids then produce ions in the water, allowing rainwater to conduct electricity a little bit. Sometimes, rain also picks up dissolved salts from dust, which also add ions.
-
-
-
Why do acids not show acidic behaviour in the absence of water?
-
Answer: Acids show their “acidic superpowers” (like turning litmus red or reacting with metals) only when they can release hydrogen ions (H⁺). They can only release these H⁺ ions when they are dissolved in water. Without water, they are just like any other compound and can’t show their acidic nature.
-
-
Five solutions A,B,C,D and E when tested with universal indicator showed pH as 4,1,11,7 and 9, respectively. Which solution is
(a) neutral? (pH 7) → Solution D
(b) strongly alkaline? (Highest pH above 7) → Solution C (pH 11)
(c) strongly acidic? (Lowest pH below 7) → Solution B (pH 1)
(d) weakly acidic? (pH below 7 but closer to 7) → Solution A (pH 4)
(e) weakly alkaline? (pH above 7 but closer to 7) → Solution E (pH 9)
Arrange the pH in increasing order of hydrogen-ion concentration.-
Answer: More H⁺ ions means more acidic, which means a lower pH. So, to arrange in increasing order of H⁺ ions, you need to arrange in decreasing order of pH.
-
Order of increasing H⁺ concentration (decreasing pH):
C (pH 11) < E (pH 9) < D (pH 7) < A (pH 4) < B (pH 1)
-
-
Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid (HCl) is added to test tube A, while acetic acid (CH₃COOH) is added to test tube B. Amount and concentration taken for both the acids are same. In which test tube will the fizzing occur more vigorously and why?
-
Answer: The fizzing (bubbles of hydrogen gas) will happen more vigorously in test tube A (with Hydrochloric acid).
-
Why: Hydrochloric acid (HCl) is a strong acid. This means it breaks apart almost completely in water and releases a lot of H⁺ ions very quickly. Acetic acid (CH₃COOH) is a weak acid. It doesn’t break apart as much, so it releases fewer H⁺ ions at a time. More H⁺ ions reacting with magnesium means a faster, more vigorous reaction.
-
-
Fresh milk has a pH of 6. How do you think the pH will change as it turns into curd? Explain your answer.
-
Answer: When fresh milk (pH 6, slightly acidic) turns into curd (dahi), its pH will decrease (it will become more acidic).
-
Explanation: Bacteria in milk (like Lactobacillus) convert the lactose (milk sugar) into lactic acid. Since acid is being formed, the pH goes down.
-
-
A milkman adds a very small amount of baking soda to fresh milk.
(a) Why does he shift the pH of the fresh milk from 6 to slightly alkaline?-
Answer: Baking soda is basic. Adding a little bit of it to fresh milk (pH 6) makes the milk slightly alkaline (pH goes a bit above 7). He does this to prevent the milk from souring (turning into curd) too quickly. The slightly alkaline environment slows down the growth of bacteria that produce lactic acid.
(b) Why does this milk take a long time to set as curd? -
Answer: Curd forms when bacteria produce enough lactic acid to lower the pH. Since the milkman made the milk slightly alkaline, the bacteria first have to neutralize this added alkalinity and then produce enough extra acid to make it sour and set into curd. This whole process takes a longer time.
-
-
Plaster of Paris should be stored in a moisture-proof container. Explain why?
-
Answer: Plaster of Paris reacts with moisture (water from the air). When it gets wet, it slowly turns into gypsum, which is a hard, solid mass. If you store Plaster of Paris in a container that isn’t moisture-proof, it will absorb moisture, harden up, and become useless for making casts or molds.
-
-
What is a neutralisation reaction? Give two examples.
-
Answer: A neutralisation reaction is when an acid reacts with a base to form salt and water. They “neutralize” or cancel out each other’s effects.
-
Examples:
-
Hydrochloric acid (acid) + Sodium hydroxide (base) → Sodium chloride (salt/table salt) + Water
HCl + NaOH → NaCl + H₂O -
Sulphuric acid (acid) + Potassium hydroxide (base) → Potassium sulphate (salt) + Water
H₂SO₄ + 2KOH → K₂SO₄ + 2H₂O
-
-
-
Give two important uses of washing soda and baking soda.
-
Answer:
-
Washing Soda (Sodium carbonate):
-
Cleaning agent: Used in detergents for washing clothes.
-
Removing permanent hardness of water: Makes hard water soft so soap works better.
(Other uses: making glass, soap, paper)
-
-
Baking Soda (Sodium hydrogen carbonate):
-
Baking: Used in cakes and bread to make them fluffy (as part of baking powder, it releases CO₂ gas).
-
Antacid: Used to relieve heartburn and indigestion by neutralizing stomach acid.
(Other uses: in fire extinguishers)
-
-
-
Acids, Bases and Salts – Multiple Choice Questions
-
A solution turns red litmus paper blue. Its pH is likely to be:
(a) 1
(b) 4
(c) 5
(d) 10 -
Which of the following is used for treating indigestion caused by excess stomach acid?
(a) Antibiotic
(b) Analgesic
(c) Antacid
(d) Antiseptic -
When dilute hydrochloric acid is added to zinc granules, which gas is produced?
(a) Oxygen
(b) Hydrogen
(c) Chlorine
(d) Carbon dioxide -
The chemical formula for Plaster of Paris is:
(a) CaSO₄·2H₂O
(b) CaSO₄·½H₂O
(c) CaCO₃
(d) CaOCl₂ -
Which of the following salts will give an acidic solution when dissolved in water?
(a) NaCl
(b) CH₃COONa
(c) NH₄Cl
(d) K₂SO₄ -
The process of electrolysis of an aqueous solution of sodium chloride (brine) is called:
(a) Neutralisation
(b) Saponification
(c) Chlor-alkali process
(d) Haber process -
What happens when a solution of an acid is mixed with a solution of a base in a test tube?
(i) The temperature of the solution increases.
(ii) The temperature of the solution decreases.
(iii) The temperature of the solution remains the same.
(iv) Salt formation takes place.
(a) (i) only
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (i) and (iv) -
Which of the following is NOT a property of a base?
(a) Turns red litmus blue
(b) Tastes bitter
(c) Feels soapy
(d) Reacts with carbonates to produce CO₂ -
Tooth enamel is made up of:
(a) Calcium carbonate
(b) Calcium phosphate (hydroxyapatite)
(c) Calcium chloride
(d) Calcium sulphate -
Baking soda is chemically known as:
(a) Sodium carbonate
(b) Sodium hydrogencarbonate
(c) Sodium chloride
(d) Sodium hydroxide -
Which gas is evolved when acids react with metal carbonates?
(a) Hydrogen
(b) Oxygen
(c) Carbon dioxide
(d) Nitrogen -
A solution has a pH of 9. It is:
(a) Strongly acidic
(b) Weakly acidic
(c) Neutral
(d) Weakly basic -
The “water of crystallisation” in Copper Sulphate (CuSO₄·5H₂O) is:
(a) 1 molecule
(b) 2 molecules
(c) 5 molecules
(d) 10 molecules -
Which of the following does NOT show acidic character in the absence of water?
(a) HCl gas
(b) H₂SO₄ liquid
(c) CH₃COOH liquid
(d) All of the above -
The common name for CaOCl₂ is:
(a) Baking soda
(b) Washing soda
(c) Bleaching powder
(d) Quick lime
Answers:
-
(d) 10
-
(c) Antacid
-
(b) Hydrogen
-
(b) CaSO₄·½H₂O
-
(c) NH₄Cl (Salt of strong acid HCl and weak base NH₄OH)
-
(c) Chlor-alkali process
-
(d) (i) and (iv) (Neutralisation is exothermic and forms salt)
-
(d) Reacts with carbonates to produce CO₂ (This is a property of acids)
-
(b) Calcium phosphate (hydroxyapatite)
-
(b) Sodium hydrogencarbonate
-
(c) Carbon dioxide
-
(d) Weakly basic
-
(c) 5 molecules
-
(d) All of the above (Acids show their properties due to H⁺ ions formed in aqueous solution)
-
(c) Bleaching powder